bioSHARE
bioSHARE: Collection of biomarkers in SHARE
Biomarker analyses have become one of the most important innovations in micro data collections in major surveys on ageing. Biomarkers improve the measurement of health status by providing information on health conditions that are not easily observed and reported by respondents. They also help to elucidate pathways connecting social and environmental factors to broad health outcomes. Biomarkers obtained from the blood include cytokines, immune system markers, molecular and cellular markers of aging, and gene expression. Coded biomarkers are available as restricted-access data linked to main longitudinal data for each respondent while the assays are stored in a long-term repository to take advantage of future scientific and technological developments.
Biomarkers have been collected in all major longitudinal ageing surveys. The Health and Retirement Study (HRS) and the English Longitudinal Study of Ageing (ELSA) collected biomarker data in 2014-2016, including venous blood samples. Twelve countries in SHARE-ERIC collected dried blood spots in wave 6 in 2014/15. Unfortunately, the Czech Republic could not join in this biomarker collection due to financial constraints and legal restrictions on fieldwork implemented by certified medical staff.
Collection of Biomarker Data in the Czech Republic: A Synergy of SHARE-CZ and RECETOX
In order to redress this gap in Czech SHARE data, a synergy between two large research infrastructures, SHARE-CZ and RECETOX at the Masaryk University in Brno, is planned for SHARE wave 11 in 2027. In full compliance with the legislation, in the Czech Republic biological samples will be collected from all respondents through sub-contracted medical facilities and analyzed by professional laboratory staff at RECETOX. Coded data will be merged with the longitudinal SHARE-ERIC data. The collection and methodology will be compatible with HRS, ELSA, SHARE-ERIC other biological sample collections. This new database, linked also to the SHARE Harmonized Cognitive Assessment Protocol (HCAP) study, will represent a unique database not only in the SHARE-ERIC but also in European and world research. This synergy will put Czech SHARE data on the forefront of world research connecting biomedicine and social sciences, opening opportunities for Czech researchers in scientific outcomes and publications.
In the planned biomarkers collection, the most important samples are venous blood (VBS) and dried blood spots (DBS). Additional measured data will include the following variables: weight, height, waist circumference, hip circumference; blood pressure and pulse; ECG electrocardiography (by a portable ambulatory machine by CardioSecur); vision, hearing, smell, speech, and balance tests; TBC vaccination mark on shoulder; lung functions as forced vital capacity (FVC), forced expiratory volume (FEV), and/or peak flow (PF). In addition, respondents will be asked to bring their medications that will be scanned by QR/barcode technology, photographed, and listed. These are the essential biomarkers that can be collected in SHARE survey conditions. As in other biomarker surveys, respondents will be informed on their test results and alerted if any of the markers is abnormal.
Biomarkers and Exposomes: In Wave 11 in 2027, all respondents in the Czech Republic will provide venous blood samples and other objective measures. Additional linkages will be provided for exposome data. Both biomarker and exposome data will be harmonized with HRS, GECC and other projects. Biomarker collection will be administered by RECETOX RECETOX Laboratories at Masaryk University, and the future coordinator of EIRENE-ERIC.
Accelerometer Study in Wave 8 in the Czech Republic
In SHARE Wave 8, data on physical activity were collected by using accelerometers. A subsample of the panel respondents were asked to wear the device (Axivity AX3) for eight consecutive days (day and night) on their upper thigh.
The SHARE accelerometer study was conducted in ten countries: Belgium, Czech Republic, Denmark, France, Germany, Italy, Poland, Slovenia, Spain, Sweden.
A detailed description of the study is available in the SHARE Wave 8 Methodology Volume.
Raw accelerometer data is processed with ActiPASS and GGIR and resulting measures are available in the Wave 8 gv_accelerometer modules. Variables based on ActiPASS provide information on postures and activities, e.g. sitting, lying, standing, walking, as well as intensity categories, i.e. sedentary, light, moderate, and vigorous activity. Activities detected by ActiPASS are available as 1-seconds intervals. Provided metrics based on GGIR include vector magnitude (ENMO), intensity gradient, and LXMX measures. Additional datasets with information on acceleration on epoch level (5 seconds) for each participant are available.
Raw sensor data is available upon request at the SHARE-ERIC Berlin Institute.
For further details see SHARE wave 9 Release Guide.
Physical measures in all waves
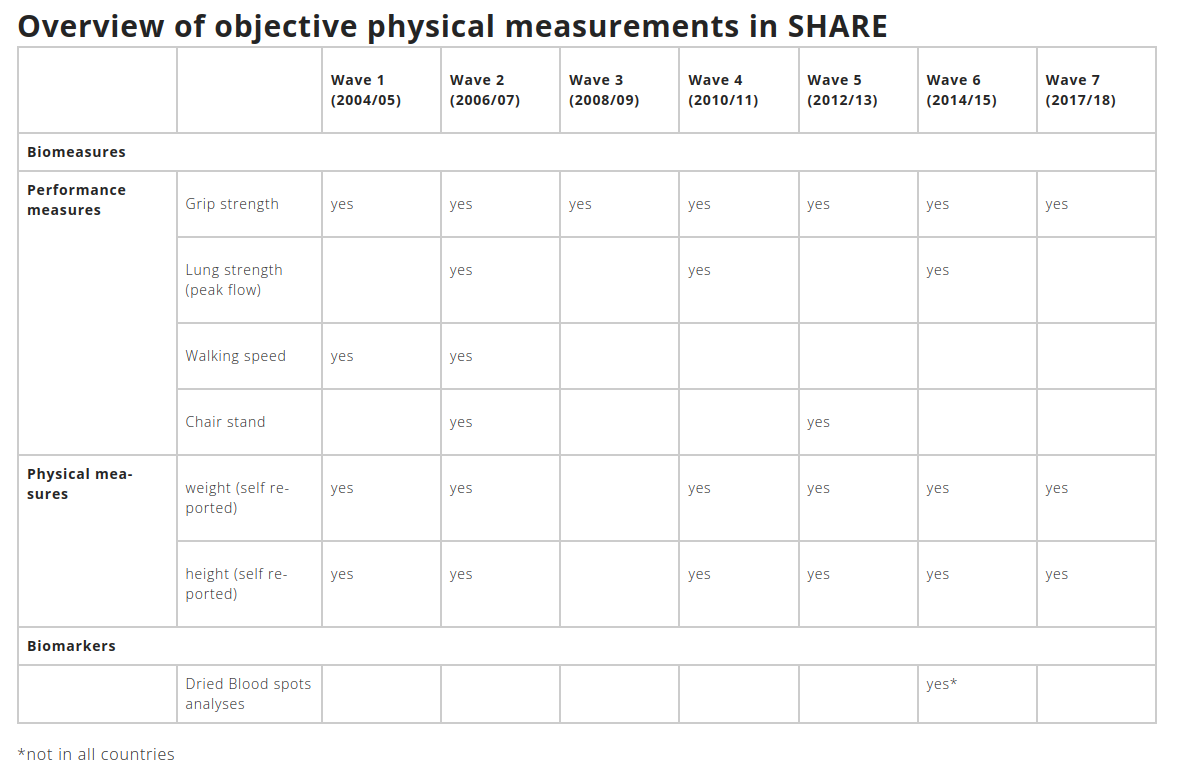
All waves of SHARE include objective health data (biomeasures) in form of physical performance measures: grip strength, walking speed (both strong predictors of future disability), peak flow (associated with health conditions, including dementia) and the so-called chair stand measurement (its result is a predictor of subsequent disability or hospitalization). Additionally, we ask respondents for their height and weight. Not all of the listed measures are collected in every wave, for more details see the table below.
SHARE Dried Blood Spots (DBS) data
Release 1-0-0 of SHARE Dried Blood Spots (DBS) data is the first release with seven blood biomarkers serving as additional objective measures of health. Twelve countries participated in the DBS sample collection during wave 6.
Dried Blood Spot (DBS) project fosters our understanding of cross-national differences in health and their causes, especially evaluating differences in health care systems, health behaviors, and historical life circumstances. Understanding differences and causes requires comparable measurements of health which do not suffer from cross-national differences in data collection and reporting styles. In this respect, analyses of blood samples became the minimum standard of objective health measurement. SHARE, the European counterpart of HRS, has therefore collected dried blood spot (DBS) samples from about 27.000 respondents aged 50 and older in twelve Continental European countries.
The project has four specific aims:
- Capitalize upon the expertise of the laboratory at the University of Washington by having the full set of DBS collected in SHARE wave 6 assayed for the lipid panel and type-2 diabetes mellitus biomarkers relevant to general health and cognitive decline.
- Link the laboratory analyses with the SHARE functional and subjective health, demographic, social, and economic data, create a user-friendly database and archive it.
- Harmonize the data obtained from the assays of SHARE DBS, ELSA VB and HRS DBS and VB.
- Use these data to perform statistical analyses on the relationships between cross-national differences in health, economic, work and social circumstances, health behaviors and health care interventions.